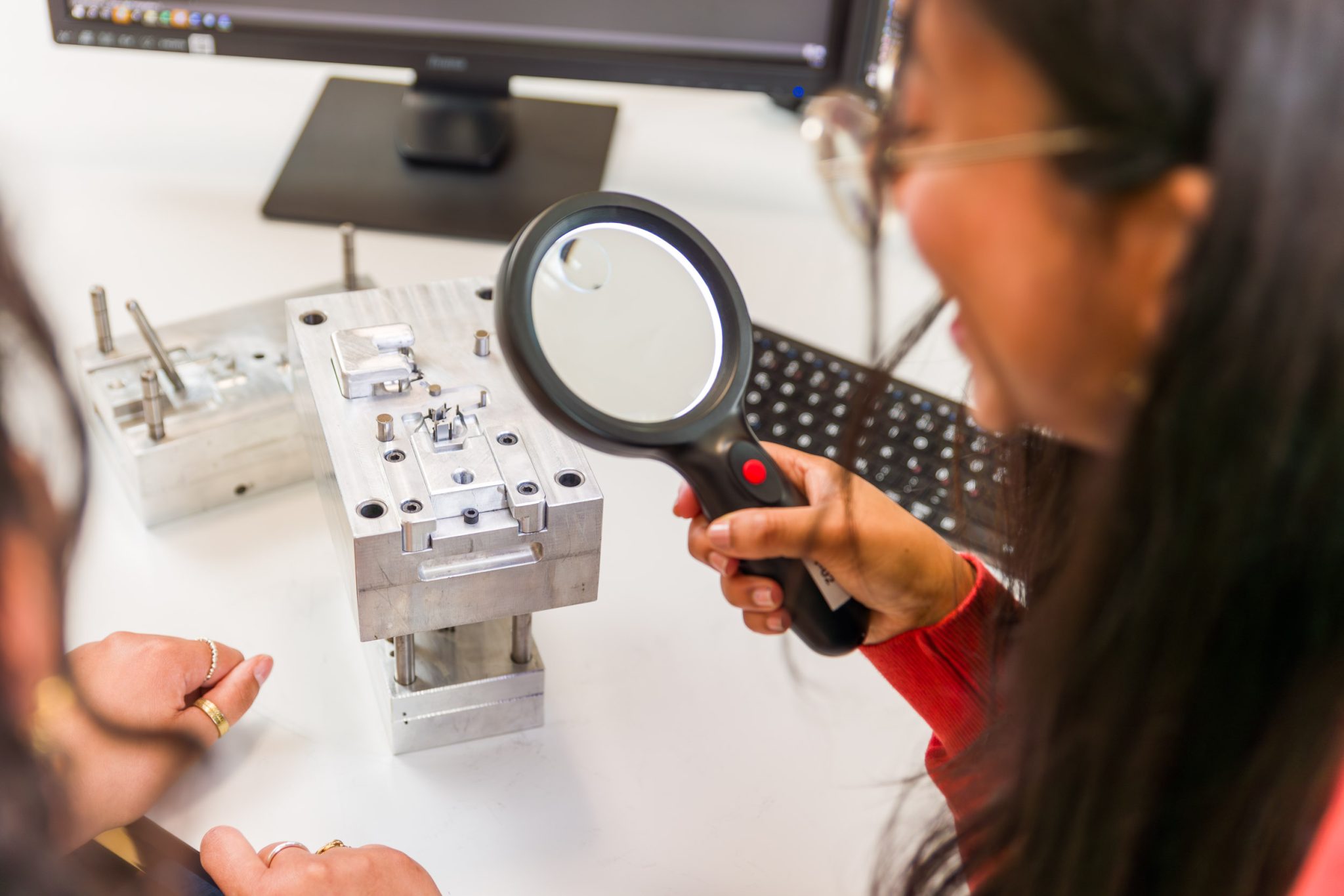
Stringent Quality Standards

Our premium quality is achieved through meticulous attention to detail. We uphold stringent standards by selecting premium materials, conducting rigorous quality checks, and maintaining precise documentation.

Our dedicated team of medical engineers, trained in Germany, ensures efficient development and continuous improvement of our products, adhering to the highest quality criteria.

Every component meets or exceeds strict German quality standards, and our manufacturing processes and quality management system are aligned accordingly to deliver superior products that meet the exacting demands of our customers.
danumed Medizintechnik implements a robust regulatory strategy at both company and product levels, ensuring optimal product quality and swift regulatory compliance and CE marking—whether for our standard products or your customized solutions.
Compliance for
Top Quality
ISO 13485 is a globally recognized standard for Quality Management Systems tailored specifically to the medical device industry. Compliance with this standard is essential for danumed as it ensures our products meet all relevant regulatory requirements, supporting their safety and effectiveness for intended use.
danumed Medizintechnik maintains a comprehensive regulatory strategy for the company and tailors specific strategies for each product and project. This approach ensures top product quality and efficient regulatory approvals, including CE marking, for both our own products and for custom solutions.

We take great pride in our accomplishments in achieving certifications across numerous countries, demonstrating our commitment to meeting rigorous regulatory standards globally.
See a choice of our achievements:
- CE mark, European Union
- ANVISA, Brazil
- Saudi FDA, Saudi Arabia
- TGA, Australia
- And many more!
Global Certifications
See a choice of our achievements:
CE mark
European Union
ANVISA
Brazil
Saudi FDA
Saudi Arabia
TGA
Australia
And many more!

Quality Assurance Through Association Activities
As a proud member of the SPECTARIS Association—a leading organization representing Germany’s high-tech sectors including analytical and medical technologies, optics, and photonics—we actively participate in industry advocacy and marketing initiatives. Our RA Manager plays a pivotal role by lending her expertise to influential working groups such as “MDR” and the “Regulatory Affairs Discussion Board Medical Technology,” ensuring our company remains at the forefront of regulatory compliance and industry standards.

Aligned With Industry Standards
Since January 2019, we have been actively involved in the DIN Standards Committee Medicine, where our experts contribute to the “NA 063-02-02 Catheters, Drainages” committee. Additionally, we are proud of our membership in the European Committee for Standardization (CEN) group “CEN/TC 205 WG 16 Catheters,” focusing on Enteral Feeding Systems’ design and testing as per ISO DIS 20695. These engagements underscore our commitment to adhering closely to industry standards and shaping the future of medical technology.
DIN, the German Institute for Standardization, is the independent platform for standardization in Germany and worldwide. As a partner for industry, research and society as a whole, DIN plays a major role in helping innovations to reach the market in areas such as the digital economy or society, often within the framework of research projects.
Experts from industry, research, consumer protection and the public sector bring their expertise to work on standardization projects managed by DIN. The result of these efforts are market-oriented standards and specifications that promote global trade, and encourage rationalization, quality assurance, and the protection of society and the environment, as well as improving security and communication.
Please see www.din.de/en for further information on DIN.